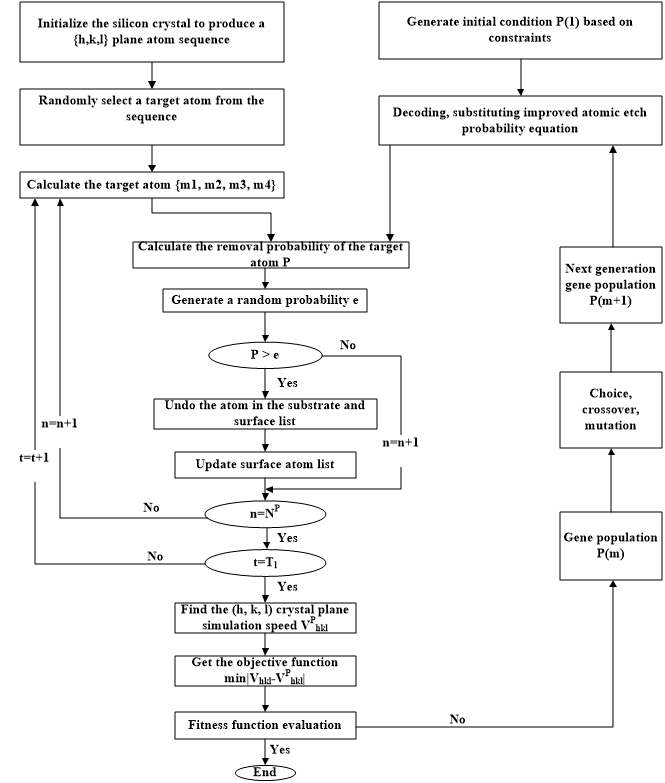
The etch model is a process used to describe the surface etching or adsorption process. The creation of the etch model is based on the plasma. Firstly, the complex physical and chemical reactions in the etching process, along with the interaction of different neutral particles and charged particles (electric field, flow field, force field, etc.), make it difficult to describe the plasma etching process. Secondly, the plasma etching process model needs to consider the process requirements corresponding to the increasing size of the chamber and the shrinking device size. Therefore, although plasma etching equipment has been widely used in the integrated circuit manufacturing industry, an effective method for completely simulating and analyzing the plasma etching process in theory has not been developed.
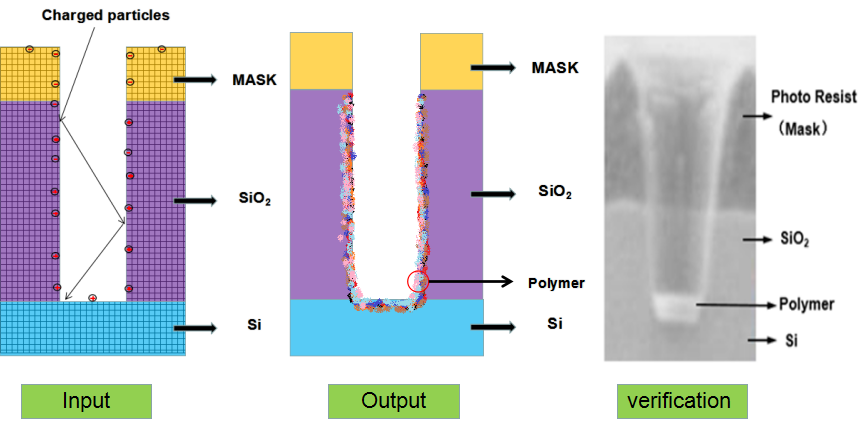
As shown in Figure 1, an etch model is created. First of all, a mathematical model of the surface feature profile is established based on the characteristics of the etching process. Then thenumerical simulation is carried out. Meanwhile the experimental conditions are selected according to the mathematical model, the silicon wafer experiment is carried out, the simulation values are fitted with the experimental values, and the mathematical model established in the previous stage is calibrated. Finally, the mathematical model and optimization algorithm are combined to improve the prediction accuracy and effectiveness of the etch model.
Figure 1.
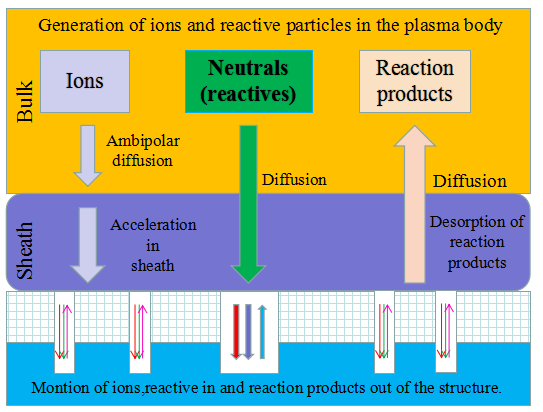
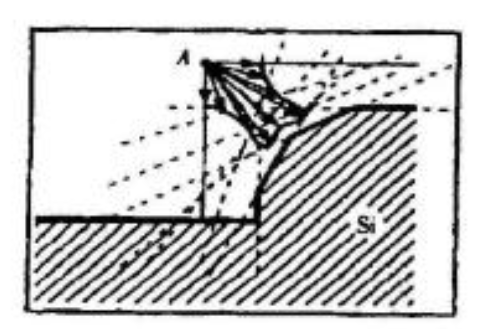
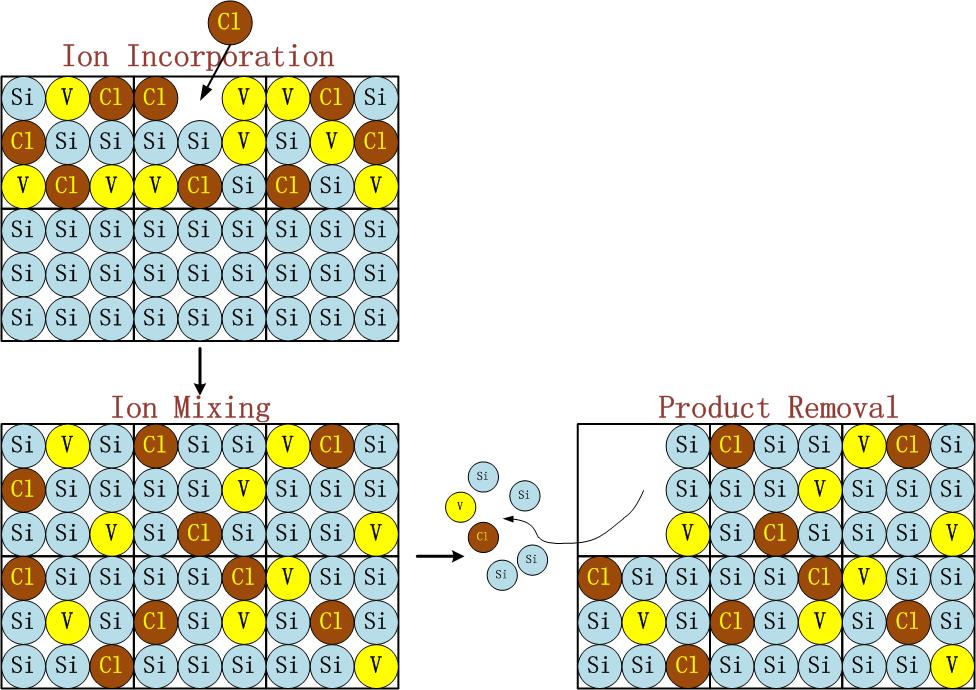
Schematic diagram of the establishment and verification of the etching model. The etching process is to transfer the photolithographic pattern onto the film on the surface of the silicon wafer. The photoresist is used to cover the wanted area while exposing the unwanted area by chemical reaction or physical reaction to complete the pattern transfer. The plasma etching is a plasma-based process and is the most widely used dry etching method. It is a general term for etching all available plasmas. The most common etching methods are reactive ion etching (RIE), inductively coupled plasma (ICP) etching, and capacitively coupled plasma (CCP) etching, etc. The chemical etching and physical etching are the two major methods in the plasma etching process, however the physical etching accounts for a small proportion. As shown in Figure 2, the plasma etching occurs in the reaction chamber. The gas is introduced into the chamber and plasma is generated during the glow discharge. By applying the accelerating electric field perpendicular to the silicon wafer, a large amount of electrons are perpendicularly colliding on the surface of the silicon wafer, and part of the kinetic energy is dissipated by the physically etching, and the remaining energy leads to the surface chemical reactions forming a volatile product, which is eventually pumped away by the vacuum system. The "reaction-peel-discharge" is repeated, and the surface of the material is gradually etched to a specified depth.
Figure 2.
Basic mechanisms. 2.1.
Establishing Factor of Etching Model
Due to the complexity of the etching process, the process conditions and parameters are difficult to set up, the process involves the intersection of microfluidics, micro-heat transfer, molecular dynamics and other disciplines.
The establishment of a perfect etching model requires consideration of various factors, including the process condition variations, corrosion interface reactions, external environment impact on the corrosion process, and the process equipment influence
[6].
2.2.
Etching Model Description Object
The etching process is described mainly three aspects: material characteristics, structural features and process characteristics.
2.3.
Method for Establishing Etching Process Model
The best approach is to start building the model from a simple one. From the physics point of view, it is preferred to assume a simple reactor shape, a single excitation method (RIE, CCP, ICP, ECR, etc.), and, a limited species of gas. Then continue to increase the parameters to optimize the model. The current etch model modeling cannot achieve good versatility because whenever the gas changes, the shape of the reactor changes or a different excitation mode is selected, the mechanism of the entire etch is changed, resulting in different etching models. The current modeling methods are as follows: (1) analytical method: this model explains the reaction mechanism and factors involved in the process and their influence on the corrosion process in the form of mathematical equations, it obtains the required parameters as input and output results by equation calculations; (2) geometry method: based on the geometric model, the feature process parameters or feature process models are used to obtain the output of the three-dimensional model through feature recognition; (3) system identification method: the neural network model is applied to establish a non-linear mapping relationship between input and output to predict the process result; (4) basic principle simulation method: based on the basic principle, a plasma etching model is established, which involves the continuity in the high-frequency, high-intensity electric field, the beam balance and the energy balance equation; (5) empirical model: in this case the basic physical process is largely ignored, and the problem is only parameterized from the perspective of actual process behavior. A mathematical model is established by analyzing how the input measurement projects to the output measurement.